The transformation of wine into vinegar is one of nature's most fascinating biochemical processes, a phenomenon humans have harnessed for millennia to create this versatile condiment. At its core, this metamorphosis hinges on the oxidation of ethanol into acetic acid, a reaction mediated by acetic acid bacteria. While the chemistry appears straightforward, the interplay of microorganisms, environmental factors, and time creates a complex dance that turns spoiled wine into culinary gold.
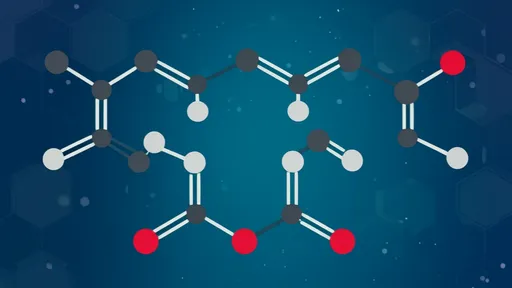
The Science Behind the Sour: When wine gets exposed to oxygen, acetic acid bacteria (primarily Acetobacter and Gluconobacter species) initiate a two-stage fermentation process. First, these aerobic bacteria convert ethanol (C₂H₅OH) into acetaldehyde (CH₃CHO) using the enzyme alcohol dehydrogenase. In the second step, aldehyde dehydrogenase oxidizes acetaldehyde into acetic acid (CH₃COOH), the compound responsible for vinegar's characteristic bite. This exothermic reaction explains why traditional vinegar production often feels warm to the touch—it's literally releasing heat as it transforms.
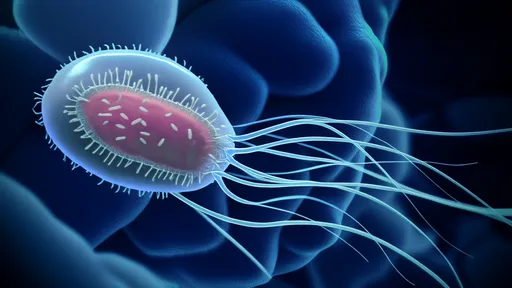
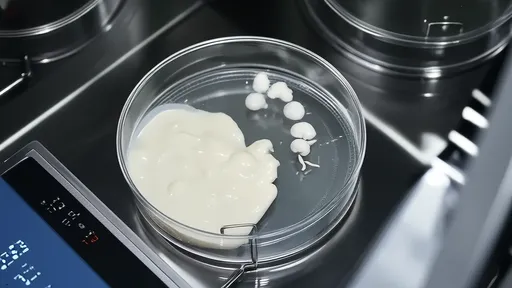
Interestingly, not all acetic acid bacteria behave identically. Acetobacter aceti dominates commercial vinegar production due to its higher alcohol tolerance and efficiency, while Gluconobacter oxydans tends to thrive in lower-alcohol environments. The bacterial cellulose mats that form during fermentation—sometimes called "mother of vinegar"—serve as both living quarters for these microbes and physical evidence of the ongoing biochemical activity.
Environmental Alchemy: Temperature plays a crucial role in vinegar production. Between 20-30°C (68-86°F), bacterial activity peaks, with warmer temperatures accelerating the process but risking the production of off-flavors. Traditional methods like the Orleans process (where wine slowly acetifies in wooden barrels over months) contrast sharply with modern industrial techniques that force oxygen through submerged cultures to complete fermentation in mere hours.
The vessel material also influences the final product. Oak barrels, still used in premium balsamic vinegar production, allow micro-oxygenation and contribute tannins that round out vinegar's sharpness. Stainless steel reactors, preferred for mass production, offer precise control but lack the flavor complexity developed through slower, traditional methods.
From Grapes to Gourmet: Wine composition directly impacts the resulting vinegar's quality. Tannins, residual sugars, and phenolic compounds from grape skins all survive the transformation, explaining why premium wine vinegars command higher prices. A vinegar made from oxidized Cabernet Sauvignon will retain the grape's dark fruit characteristics, while Champagne vinegar carries delicate floral notes impossible to replicate with cheaper alcohol sources.
Artisanal producers often blend wines before acetification to create signature flavors. Some add dried fruits or herbs during fermentation, a technique called maceration that infuses additional complexity. The finest balsamic vinegars undergo years of aging in progressively smaller barrels, concentrating flavors through evaporation—a process not unlike the solera system used for premium sherries.
The Microbial Tightrope: Successful vinegar production walks a fine line between controlled spoilage and actual spoilage. While acetic acid bacteria are desirable, contamination by other microbes can ruin a batch. Lactic acid bacteria may create off-flavors, while certain yeasts produce cloudiness or unwanted carbonation. This explains why traditional vinegar makers guarded their bacterial cultures as closely as bakers protect their sourdough starters.
Modern quality control measures include pasteurization to stabilize vinegar after fermentation, though this kills the beneficial bacteria prized in raw, unfiltered products. The ongoing debate between traditionalists and industrial producers often centers on this very point—whether to prioritize microbial diversity or shelf stability.
Beyond the Kitchen: The acetic acid produced through this biochemical process finds uses far beyond salad dressings and pickling brines. Historically, vinegar served as a medicinal antiseptic (Hippocrates prescribed it for wound cleaning) and food preservative. Today, derivatives of acetic acid appear in plastics production, photographic chemicals, and even as a household cleaner—all originating from the same bacterial process that turns forgotten wine into something extraordinary.
As research continues into acetic acid bacteria's full potential, scientists are exploring applications in biofuel production and wastewater treatment. The same metabolic pathways that create vinegar might someday help address energy and environmental challenges—proving that this ancient biochemical process still holds modern relevance.
From accidental discovery to intentional craftsmanship, the journey from wine to vinegar represents one of humanity's earliest successes in harnessing microbial biochemistry. Whether produced in a French cooper's barrel or a high-tech bioreactor, this tart transformation remains a testament to nature's ability to create value from what might otherwise be considered waste—a delicious alchemy that continues to evolve.
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025