The warm, sweet aroma of star anise has captivated cooks and chemists alike for centuries. Behind its distinctive flavor lies a fascinating molecule called anethole, the chemical cornerstone of this iconic spice. This organic compound not only defines the characteristic licorice-like taste of star anise but also reveals intriguing connections across botany, gastronomy, and even traditional medicine.
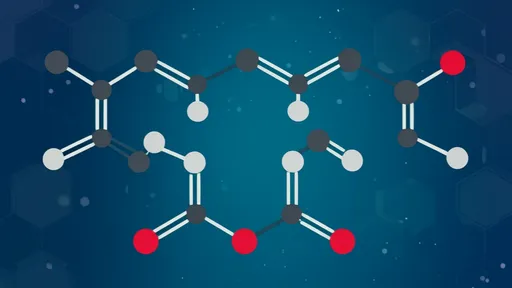
Anethole belongs to a class of compounds known as phenylpropenes, aromatic molecules commonly found in plants. Its structure consists of a benzene ring connected to a propene tail with a methoxy group (-OCH3) at the para position. This seemingly simple arrangement creates a remarkably versatile molecule that exists in two isomeric forms: the more common trans-anethole and the less stable cis-anethole. The trans configuration predominates in nature and is responsible for the familiar sweet flavor, while the cis form tends to be bitter and less aromatic.
The chemistry of anethole explains its unique behavior in food preparation. Being relatively non-polar, it dissolves readily in oils and alcohol but poorly in water. This property becomes evident when making anise-flavored liqueurs like ouzo or pastis – the clear liquid turns milky when diluted with water due to the precipitation of anethole crystals. Chefs have long exploited this characteristic to create dramatic visual effects in cocktails and sauces.
Beyond star anise (Illicium verum), anethole appears in various botanicals including fennel, basil, and tarragon. However, the concentration and accompanying compounds differ significantly between species. Star anise contains particularly high levels of anethole (80-90% of its essential oil composition), along with smaller amounts of limonene, pinene, and other terpenes that modify its flavor profile. This chemical composition varies depending on growing conditions, harvest time, and processing methods, creating subtle differences in flavor between batches.
The extraction of anethole from star anise involves either steam distillation or solvent extraction. Traditional methods used in Chinese medicine involve prolonged soaking in alcohol, while modern industrial processes employ more efficient techniques. Interestingly, synthetic anethole can be produced from petroleum derivatives, though discerning palates often detect subtle differences compared to the natural version. The debate between natural and synthetic flavors continues among food scientists and gourmets.
In the kitchen, anethole's stability under heat makes it invaluable for cooking. Unlike many volatile aromatics that dissipate quickly, anethole maintains its character during prolonged simmering, explaining why star anise features prominently in braised dishes and slow-cooked broths. Its flavor develops and mellows over time, creating complex depth in dishes like Vietnamese pho or Chinese master stock. Professional chefs have learned to balance its potency with other spices to avoid overwhelming delicate ingredients.
The biological function of anethole in plants offers another layer of fascination. Researchers believe it serves as both a defense chemical against herbivores and a potential attractant for specific pollinators. Some studies suggest that the compound may have antimicrobial properties, which could explain its traditional use in food preservation. Ancient cultures seemed to intuitively recognize these properties long before modern science could verify them.
Modern research has uncovered potential medicinal applications for anethole beyond its culinary uses. Preliminary studies indicate possible antioxidant, anti-inflammatory, and even estrogen-like effects, though conclusive clinical evidence remains limited. Traditional medicine systems have employed star anise for digestive issues and respiratory conditions for centuries, with anethole likely contributing to these therapeutic effects. However, scientists caution against self-medication due to potential toxicity at high doses.
The flavor chemistry of anethole extends to surprising pairings. While classically associated with sweet applications, it can enhance savory dishes when used judiciously. Molecular gastronomy experiments have revealed its affinity for certain proteins and fats, explaining why anise flavors complement rich meats like duck and pork. Contemporary mixologists have also rediscovered anethole's versatility, creating innovative cocktails that play on its sweet-aromatic character.
As consumers increasingly seek natural flavorings and functional foods, anethole's star continues to rise. Food manufacturers carefully monitor sourcing as global demand grows, with sustainability concerns prompting research into cultivation practices. Climate change may affect future harvests, as star anise trees require specific conditions to produce optimal anethole concentrations. These factors combine to make this humble molecule far more than just a flavor compound – it's a lens through which we can examine cultural, scientific, and economic intersections.
From its role in ancient pharmacopeias to its place in modernist cuisine, anethole's journey mirrors humanity's evolving relationship with nature's chemical bounty. The next time you savor the distinctive taste of star anise, remember that you're experiencing one of nature's most elegantly designed flavor molecules – a perfect marriage of chemistry and sensory delight that has stood the test of time.
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025
By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025

By /Jul 14, 2025